

What is Electronegativity
The tendency of a bonded atom to atom to attract a shared pair of electrons is known as electronegativity. In other words, we can say that electronegativity is a measure of the tendency of an atom in a molecule to attract paired of an electron to itself.
If the ability of an atom to attract a shared pair of electrons is greater, then electronegativity will also be greater. Electronegativity is a fundamental property of an atom that is different from other properties of an atom like electron affinity. Electron affinity shows the tendency of an isolated gaseous atom to attract electrons while electronegativity is the tendency of a bonded atom to attract the shared pair of electrons between two atoms.
The ionization energy and electron affinity of an atom in a molecule are similar to electronegativity. The electronegativity depends on the structure of the atom and the number of atoms present t in a molecule. The electronegativity of small atoms is mostly greater than large atoms because the shielding effect of larger atoms is greater than small atoms.
The electronegativity of those atoms is greater having a nearly complete valence shell than the atoms having an incomplete valence shell or half-filled. For example, the electronegativity of Group 17 elements (Halogens) is greater because these elements exist with nearly filled shells while the electronegativity of Group 1-A (Alkali) is less due to scarcely filled.
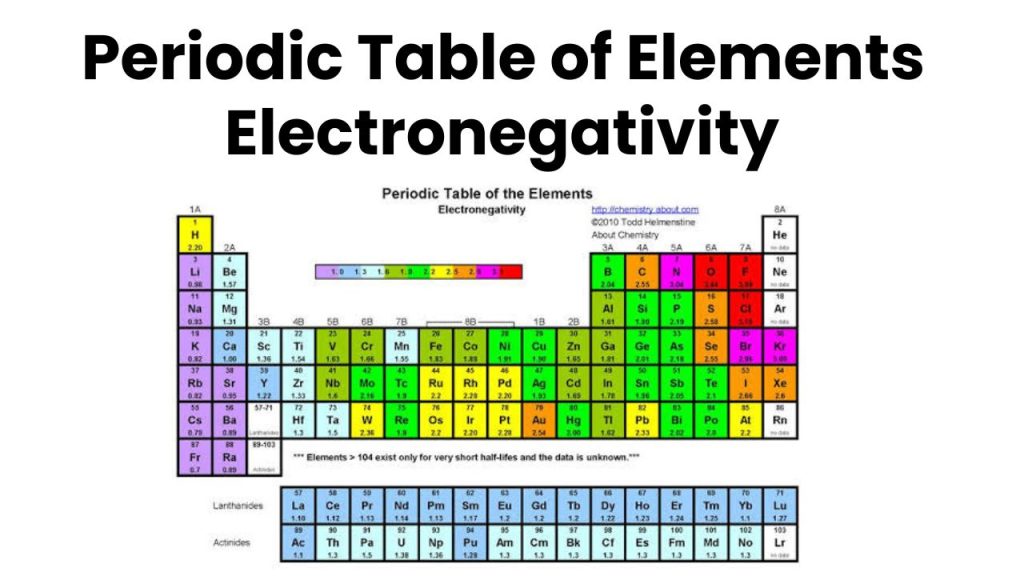
Factors Affecting the Magnitude of Electronegativity
The magnitude of electronegativity depends on the following factors.
(I) Atomic Size
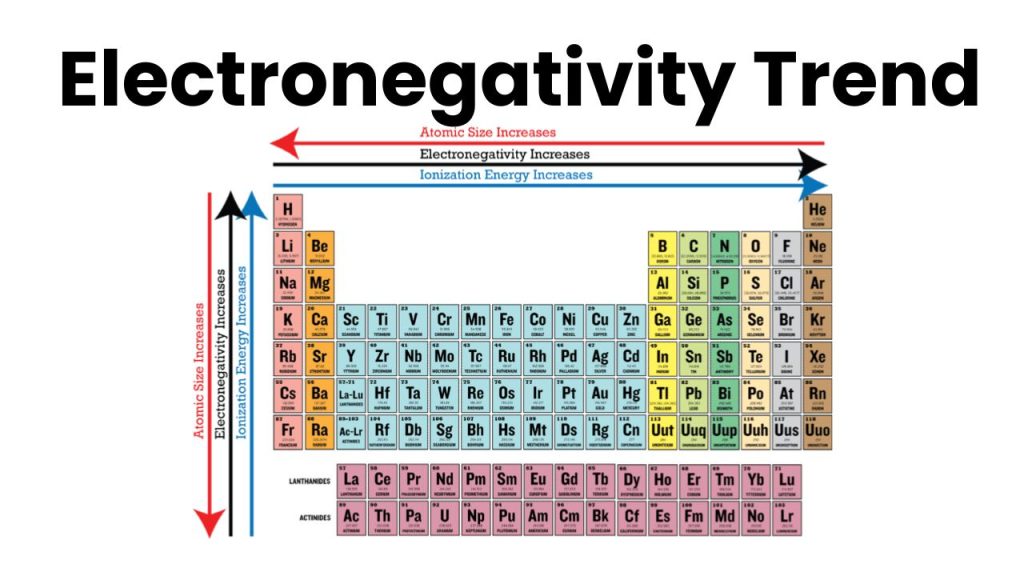
The atomic size of an atom is inversely proportional to electronegativity. If the size of an atom is greater, then electronegativity will be greater. Similarly, if the size of an atom is large then the electronegativity of an atom will be small.
(II) Number of inner shells
if the number of inner shells between the nucleus and the valence shell is greater, then electronegativity will be smaller. In the atom in which the number of the inner shell is greater, the value of electronegativity is smaller. For example, the electronegativity of group 17 (Halogen) decreases from top to bottom, because the number of inner shells increases from top to bottom. For example, the electronegativity of F is greater than the electronegativity of Iodine because the number of inner shells in iodine is greater than the number of inner shells in fluorine.
Electronegativity Trends
In the periodic table, electronegativity increases from left to right in the period because in the period size of the atom decreases. As we know the size of an atom is inversely proportional to electronegativity.
In groups from top to bottom, the electronegativity decreases because the size of the atom increases from top to bottom. For example, the electronegativity of Fluorine is greater because it is at the top of the group similarly the electronegativity of Iodine is less because it is at the bottom of the group in the periodic table.
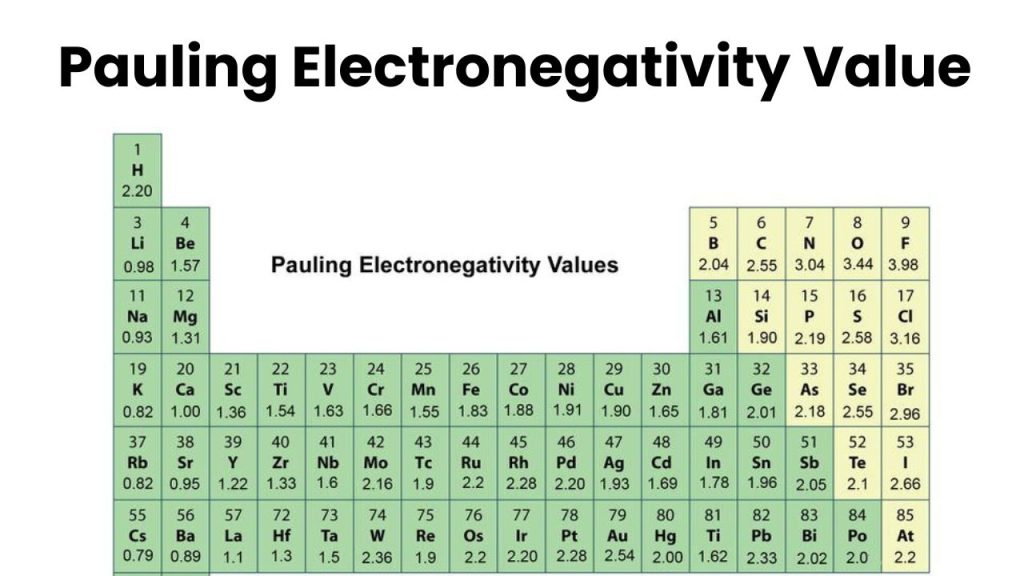
Measurement of Electronegativity
Several scales have been used to measure the electronegativity of atoms. These scales are arbitrary and the base of these scales depends on various experimental data like the energy of bon, dipole moment, ionization energy, and electron affinity of the atom. The most common scale to measure electronegativity is Mulliken’s Scales.
Mulliken’s Scales
In 1934 R.S Mulliken suggested that is electronegativity of an atom is given by a formula. The formula used to measure the electronegativity is that ionization energy minus electron affinity.
X= IE-EA/2
Until recently, only a few electron affinities had been measured. For this reason, Mulliken’s scale had limited utility.
Pauling's scale
This scale is based on the empirical relation between the electronegativities of two bonded atoms and the energy of the bond (called bond energy).
He called it electronegativity. Imagine you have two different atoms, A and B, joined together in a molecule (let’s call it A-B). Pauling figured out electronegativity by looking at how much the A-B bond energy differed from the average of the A-A and B-B bond energies. It’s like figuring out how much one atom in a pair pulls on shared electrons compared to when it’s with its kind.
EA-B = [EA-A – EB-B]1/2
Puling suggested that if the electronegativity of two bonded atoms A and B is the same then the energy of the bond between them (A-B) would be the average of the energies in bonds where A is with A and B is with B. This happens when the electrons are shared equally, like in purely covalent bonds. He expressed it like this:
Pauling observed that for the majority of A-B bonds, the energy exceeds the geometric average because generally the atoms A and B have different electronegativities and there is an ionic contribution to the bond in addition to the covalent one. Let the difference between A-B bond energy and the geometric mean of A-A and B-B be Δ,
Δ = EA-B – [EA-A x EB-B]1/2
This excess energy is known as the ionic resonance energy. Pauling proposed that this excess energy can be used as an empirical basis to determine electronegativity difference. If XA and XB represent the electronegativities of A, and B respectively, the difference XA – XB could be related to Δ as.
XA -XB = [Δ/96.5kJmol-1]1/2
The factor 96.5 kJmol-1 converts Δ from kJmol-1 to electron volts(eV) per molecule.
Orbital Electronegativity
Electronegativity is believed to depend on the type of orbital involved in the bonding. The s orbital penetrates closer to the nucleus than the p orbital, therefore the s orbital is more electronegative. The electronegativities of the orbitals therefore depend on the percentage of s and p characters in sp3, sp2, and sp hybridization.
The magnitude of the electronegativity increases with increasing s character in the hybrid orbital. Therefore, the electronegativities (on the Pauling scale) for the carbon atom are 2.48, 2.75, and 3.29 in sp3, sp2, and sp hybridization, respectively. The commonly used electronegativity value of 2.5 for carbon is based on sp3 Hybridization.
Group Electronegativity
The electronegativity of an atom, corrected for the presence of a substituent, is called group electronegativity. The group electronegativity of CH3 or CCl3 will be the electronegativity of carbon adjusted in the presence of three H or Cl atoms. Several methods making use of kinetic data, atomic electronegativity, and other physical measurements, have been developed to calculate the group electronegativities. Electronegativities of some common groups are listed below:
Electronegativities of some groups
Group | Electronegativities | Group | Electronegativities |
CH3 CF3 CCl3 | 2.3 3.35 3.00 | CN COOH C6H5 | 3.3 2.85 3.0 |
Solve the Quiz
FAQs
What is 14.22731 rounded to 2 decimal places?
What is 14.22731 rounded to 2 decimal places?
What is 13.412 rounded to 2 decimal places?
What is 58.8136 rounded to 2 decimal places?
Class XI Chemistry Questions
Just click To solve these chemistry practice questions. passing Marks are 50%
Class XII Chemistry Questions
Just click To solve these chemistry practice questions. passing Marks are 50%
chemistry Quiz by EXPERTS

About Us
Unlock the doors to success with a premier chemistry academy. Explore our top-notch education, experienced instructors, and a bright future in chemistry.
Pages
-
NEET Practice Question
-
JEE Main & Advance Questions
-
MDCAT Practice Questitons
Help
- Chemistry Problems
-
Chemistry Concepts
-
Chemistry Notes
